About us
Lys Therapeutics, first-in-class biotherapies against neurological diseases
Science
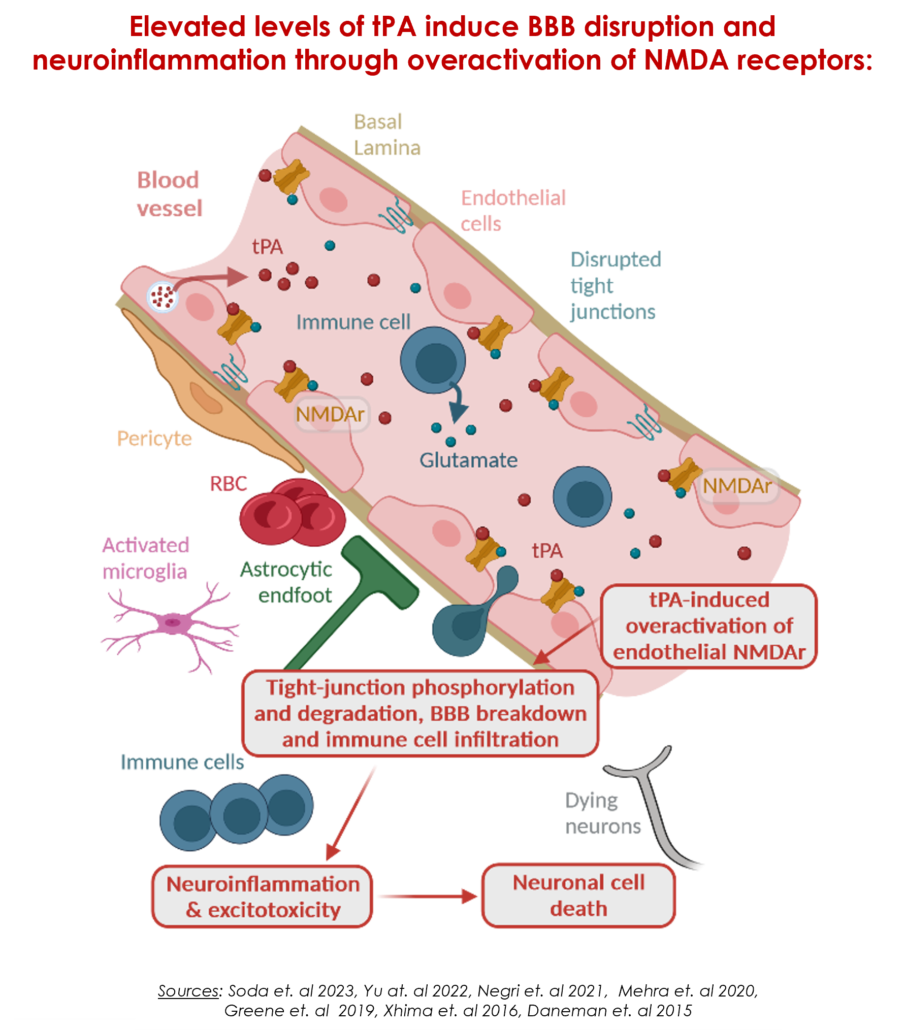
A breakthrough strategy to reverse blood-brain barrier dysfunction
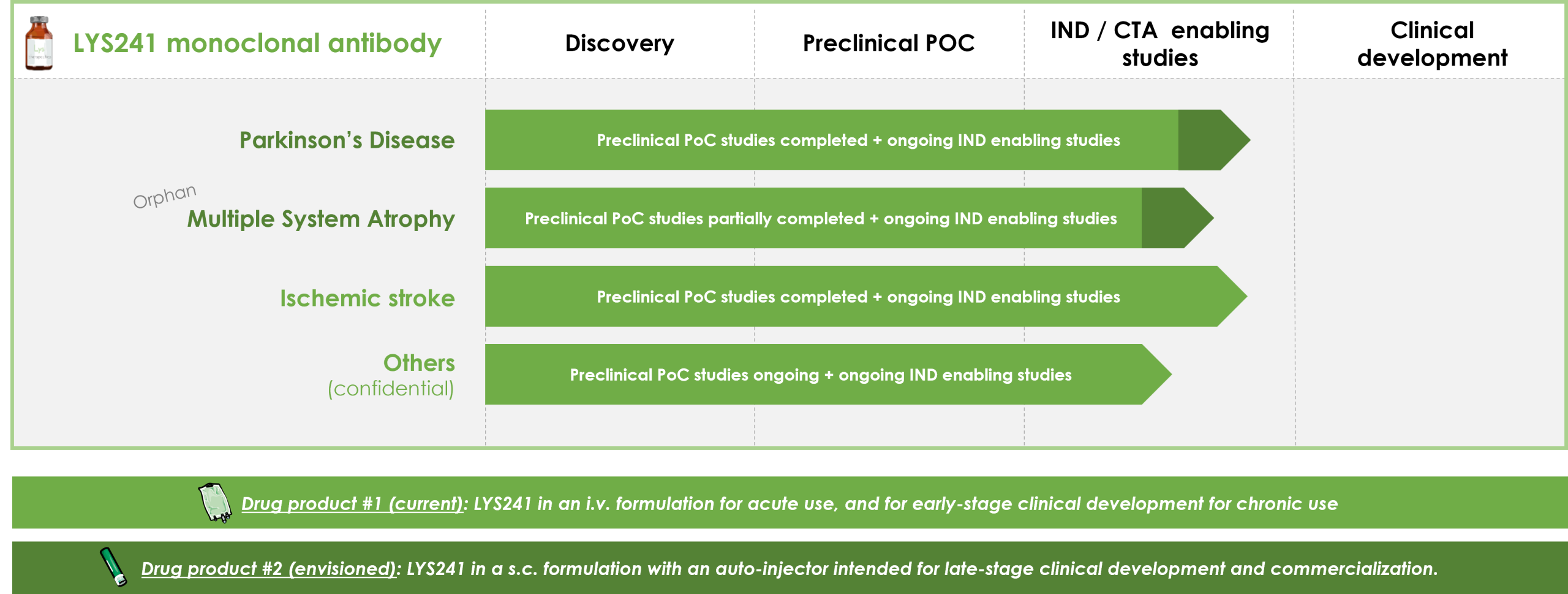
LYS241 monoclonal antibody: counteracts neuroinflammatory and neurodegenerative processes involved in the pathophysiology of neurological diseases by restoring the physiological integrity of the blood-brain barrier.
Cytoprotection approach: counteracting neuroinflammation to tackle neurodegeneration
In the pathophysiology of several neurological diseases, hyperactivation of endothelial NMDA receptors by increased expression of endogenous tissue plasminogen activator (tPA) leads to tight junction phosphorylation and degradation, and ultimately blood-brain barrier dysfunction. This allows transmigration of inflammatory cells and toxic molecules into the brain parenchyma as well as activation of microglia, resulting in severe neuroinflammation, a primary cause of neuronal cell death and disease progression.
Created with BioRender.com
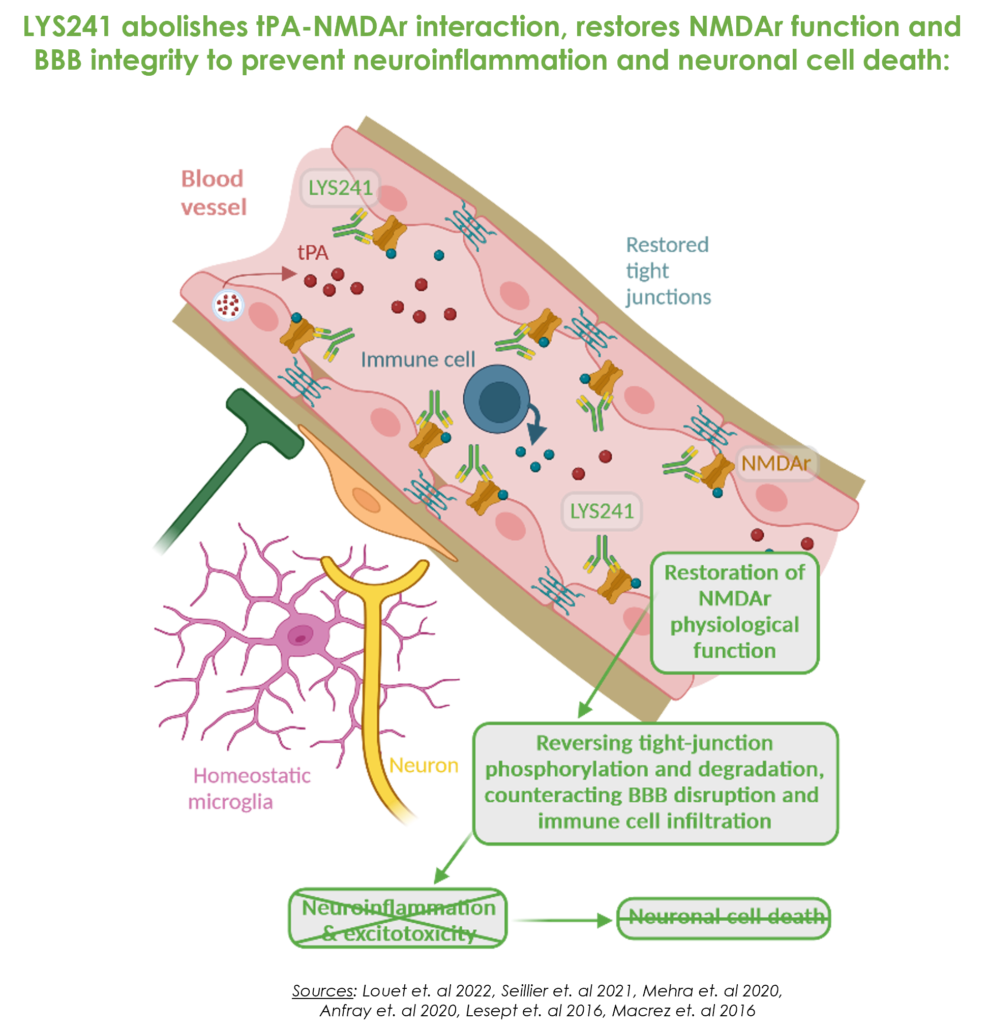
A unique mechanism of action: a CNS drug which does not need to cross the blood-brain barrier
Lys Therapeutics’ main drug-candidate, LYS241, is a first-in-class monoclonal antibody displaying a groundbreaking mechanism of action counteracting these mechanisms by specifically preventing inside blood vessels the binding of tPA on NMDA receptors, without blocking the physiological function of NMDAr.
By inhibiting this interaction, NMDAr can operate normally, haltering downstream deleterious cellular pathways. Tight junctions are reestablished, endothelial cells return to their healthy state and the blood-brain barrier function is restored, protecting the brain from further neuroinflammatory and subsequent neurodegenerative cascades.
Scientific foundations & publications:
POC studies in Stroke
POC studies in Multiple Sclerosis
Meet our team
Dr. Manuel Blanc, PhD, MBA
Co-founder, Chief Executive Officer and President
Board member
Philippe Dujardin
Co-founder, Chief Financial Officer
Board member
Pr. Denis Vivien, PhD, PU-PH
Head of Science, President of the Scientific Advisory Board
Riad Abes, PhD
Chief Development Officer
Dr. Caroline Ballet, PhD
Program Manager, Non-Clinical Operations
Dr. Jean-Baptiste Bertrand, PhD
Head of Clinical Operations
Dr. Nathalie Delétage, PhD
Senior Program Manager, Preclinical & Clinical R&D
Jessy Dorange, MSc
Technician, Preclinical R&D
Dr. François Fossiez, PhD
Head of CMC
Dr. Jordan Guyon, PharmD, MBA
Chief Operating Officer
Sylvain Hemery
Technician, Preclinical R&D
Werner Klinger
Head of IT and data protection
Dr. Flavie Lesept, PhD
Program Manager, Preclinical R&D
Dr. Fanny Potzeha, PhD
Project & Lab Manager, Preclinical R&D
Ambre Vivet-Gros
Accounting/HR assistant
Board of Directors
Michel Vounatsos, MBA
Chairman of the Board, independant member
Anat Naschitz, LLB, MBA
Member of the Board
Philippe Bissay, MBA
Member of the Board, HAC Future representative
Dr. Shibeshih Belachew, MD, PhD
Member of the Board
Scientific Advisory Board
Neurovascular diseases
President of the SAB: Pr. Denis Vivien (PhD, PU-PH), France, co-inventor of glunomab & expert of tPA, head of the Research Institute “Blood and Brain @ Caen-Normandy”
Pr. Joan Montaner (MD, PhD), Spain, neurologist, head of Neurovascular Research at the Vall d’Hebron Research Institute
Pr. Richard Macrez (MD, PhD), France, co-inventor of Glunomab, Professor and emergency physician, head of the Emergency Unit at Caen-Normandy Hospital
Dr. Désiré Collen (MD, PhD), Belgium, inventor of rtPA (alteplase) still the most effective drug for thrombolytic therapy of acute myocardial infarction and ischemic stroke
Pr. Yannick Béjot (MD, PhD), France, neurologist, head of Neurology at Dijon-Bourgogne hospital
Via BB@C Institute: Pr. Eng Lo (MD, PhD), USA, neurologist, Professor of Neurology and Radiology, Harvard Medical School, and Research Staff, Massachusetts General Hospital
Via BB@C Institute: Pr. Martin Dichgans (MD, PhD), Germany, neurologist, Director of the Institute of Stroke and Dementia Research (Medical Center of the University of Munich), head of the World Stroke Organization
Scientific Advisory Board
Neurodegenerative disorders
Pr. Werner Poewe (MD), Austria, Director of the Department of Neurology at Innsbruck Medical University in Innsbruck. Main research interests in the field of Parkinson’s disease and movement disorders. Professor Listed among top 1% of ‚highly cited researchers‘ in neuroscience.
Pr. Olivier Rascol (MD, PhD), Professor of Clinical Pharmacology at the University Hospital in Toulouse, France and Chair of the Movement Disorder Society-European Section (MDS-ES). Member of the editorial board of Lancet Neurology, Neurology and the European Journal of Neurology.
Pr. Pierre-Olivier Couraud (PhD), France, Head of the Institut Cochin (INSERM, CNRS, University of Paris, France), Chairman of the INSERM Scientific Advisory Board and Vice President of the French multiple sclerosis foundation ARSEP.
Career
We don’t have open positions at the moment, but stay tuned!